What Animals Are Hox Genes Found In
As mentioned throughout this volume, the expression of Hox genes provides the ground for inductive-posterior axis specification throughout the animate being kingdom. This means that the enormous variation of morphological class in the animal kingdom is underlain by a mutual prepare of instructions. Indeed, one of the most remarkable pieces of evidence for deep homologies among all the animals of the world is provided past the Hox genes. As mentioned in Chapter xi, not simply are the Hox genes themselves homologous, but they are in the same order on their respective chromsomes. The expression patterns are also remarkably similar between the Hox genes of unlike phyla: the genes at the 3′ end are expressed anteriorly, while those at the 5′ stop are expressed more posteriorly (Figure 22.2). As if this evidence of homology were non plenty, Malicki and colleagues (1992) demonstrated that the human being HOXB4 gene could mimic the function of its Drosophila homologue, deformed, when introduced into Dfd-deficient Drosophila embryos. Slack and his colleagues (1993) postulated that the Hox factor expression pattern defines the development of all animals, and that the pattern of Hox cistron expression is constant for all phyla.*
If the underlying Hox cistron expression is uniform, how did the differences among the phyla emerge? It is thought that they arose from differences in how the Hox genes are regulated and what target genes the Hox-encoded proteins regulate. Gellon and McGinnis (1998) have catalogued four critical means in which variation in Hox expression patterns might lead to evolutionary modify (Figure 22.3):

Figure 22.3
Changes in Hox genes correlate with evolutionary changes in animal morphology. (A) Changes in the number of Hox genes correlate with changes at the level of phyla, and increasing the number of Hox genes may allow increased complexity. (B) Broad changes (more...)
-
Changes in the Hox poly peptide-responsive elements of downstream genes
-
Changes in Hox cistron transcription patterns within a portion of the body
-
Changes in Hox gene transcription patterns between portions of the body
-
Changes in the number of Hox genes
Changes in Hox-responsive elements of downstream genes
1 of the near obvious differences between a fruit wing and a butterfly is that the fly has two halteres where the butterfly has a pair of hindwings (Figure 22.4). The expression pattern of the Hox genes, however, does non differ betwixt a butterfly larva and a wing larva. In both cases, the Ultrabithorax (Ubx) gene is expressed throughout the imaginal discs of the third thoracic segment (from which the hindwing and haltere are derived). What distinguishes a haltere from a wing is the response of the target genes. The Ubx protein downregulates several genes in the Drosophila imaginal discs. Many of these aforementioned genes are not regulated past Ubx in collywobbles. Moreover, some other genes regulated by Ubx in Drosophila are regulated differently in butterflies (Carroll et al. 1995; Weatherbee et al. 1999). Thus, the fly differences between dipterans (two-winged insects such as flies) and lepidopterans (butterflies and moths) can exist attributed to the dissimilar means in which potential target genes in the imaginal discs respond to the Ubx protein.

Figure 22.4
Differences in larval and developed morphology due to Hox cistron differences. (A, B) Larva and developed of Drosophila, a dipteran. An pointer points to one of the halteres of the developed. The larva lacks prolegs; its anterior terminate is at the left. (C, D) Larva and adult (more...)
Changes in Hox gene transcription patterns within a trunk portion
In Chapter 16, we saw that changes in Hox gene expression are correlated with the change in morphology from the fish fin to the tetrapod limb. Among arthropods, differences in limb morphology may besides be caused past Hox gene expression differences. Insects take half dozen legs as adults, a pair arising from each of the 3 thoracic segments. In Drosophila, the Distal-less (Dll) gene is critical for providing the proximal-distal axis of the appendages (see Figure eighteen.15). Distal-less expression occurs in the cephalic and thoracic limb-forming discs, merely information technology is excluded in the abdomen by the abdA and Ubx proteins. Thus, the appendages grow into legs and wings in the thorax and into jaws in the caput. The Drosophila larva never develops limbs in its abdomen.
Butterfly and moth larvae, yet, are characterized by rudimentary intestinal legs called prolegs (see Figure 22.4). Panganiban and her colleagues (1994) cloned the Distal-less homologue from the buckeye (Precis) butterfly and mapped its expression during development. During the early portion of Precis embryogenesis, Dll expression is the same every bit information technology is in Drosophila. During gastrulation, Dll expression is seen first in the head regions and in the thoracic regions that volition give ascent to the leg imaginal discs. However, every bit development gain, the Dll gene of Precis becomes expressed in the third through sixth abdominal segments (Effigy 22.5). Whereas Dll expression is seen in both the proximal band and the "socks" of the truthful thoracic legs, the expression of Dll in the abdomen is restricted to the proximal ring. Thus, the lepidopteran prolegs appear to exist homologous to the proximal portion of the thoracic legs. The expression of Dll in the maxilla and labial segments in both Drosophila and Precis is interesting because it is consistent with contempo paleontological bear witness (Kukalova-Peck 1992) that, although these jaw structures originated from limb primordia, distal limb elements have been lost from all arthropod jaws.

Effigy 22.5
Distal-less gene expression in the larva of the buckeye butterfly Precis. By 40% of the mode through embryonic development, Dll expression in Precis has diverged significantly from that of Drosophila in that Dll expression is also seen in abdominal segments (more...)
The presence of larval prolegs and Dll expression in the Precis intestinal segments suggests that Dll is regulated differently in dipterans and lepidopterans. Two possibilities come to the fore: first, that the Dll genes of Precis are non repressed past the abdA and Ubx homeodomain proteins, and second, that the expression of the repressing homeodomain genes is somehow abrogated in the abdominal regions of Precis. Warren and co-workers (1994) showed that the Drosophila and Precis embryos have the aforementioned initial blueprint of abdA and Ubx gene expression. Yet, at about twenty% of the way through Precis embryogenesis, the expression of these genes becomes downregulated in small patches of segments A3-A6, the intestinal segments that give rise to the prolegs (Figure 22.6). Before long thereafter, the Dll and Antennapedia (Antp) genes are expressed in those "holes." It is not known what molecules are used to downregulate abdA and Ubx cistron expression in the regions of Dll expression. The Polycomb group genes are the best suspects, since they tin can repress both genes in Drosophila.

Effigy 22.half dozen
"Holes" in the expression of abdA and Ubx in the abdomen of the larval butterfly Precis. The abdA and Ubx proteins accept been stained dark-green. The Distal-less protein is stained red, and areas of overlap appear yellowish. (A) In the early caterpillar, (more...)
Changes in Hox gene expression between body segments
The origins of maxillipeds in crustaceans
In that location are substantial differences in Hox gene expression patterns between insects and crustaceans, and there are also significant differences in Hox factor expression patterns inside the different crustacean groups. Crustaceans are characterized by a pre-gnathal head (similar to the insect acron), gnathal (jawed) head segments, six thoracic segments, genital segments, abdominal segments, and a telson (Effigy 22.vii). Each of the thoracic segments of the crustacean expresses Antp, Ubx, and abdA, and these genes appear to exist interchangeable in the crustaceans. Indeed, the thoracic segments all look alike, and there is no specialization betwixt these segments. In the arthropod lineage that gave rise to the insects, each factor would take on different (simply sometimes overlapping) functions (Averof and Akam 1995).

Figure 22.7
Hox cistron expression and morphological change in crustaceans. At the bottom are the structures of a brine shrimp (crustacean) and a grasshopper (insect). The domains of Hox gene expression specifying the various structures are coded in color. Whereas the (more...)
Just within the crustacean lineages, there are interesting variations on this theme. Averof and Patel (1997) accept shown that if a thoracic segment does not express Ubx and abdA, it converts its anterior locomotor limb into a feeding appendage called a maxilliped. Thus, brine shrimp such every bit Artemia have a compatible expression of Ubx and abdA in their thoracic segments, and they lack maxillipeds. Lobsters such equally Homaris lack Ubx and abdA expression in their first and second thoracic segments, and these segments have paired maxillipeds (Figure 22.8). The fossil tape suggests that the earliest crustaceans lacked maxillipeds and had compatible thoracic segments. This would mean that the presence of maxillipeds is a derived feature that evolved in several crustacean lineages.

Effigy 22.8
Schematic representation of the expression of Ubx and abdA (green) in the thoracic segments of unlike types of crustaceans. The generation of maxillipeds occurs in the thoracic segments that do non limited either of these homeodomain proteins. (After (more...)
Why snakes don't have legs
Every bit shown in Chapter eleven, the expression patterns of Hox genes in vertebrates determines the type of vertebral structure formed. Thoracic vertebrae, for instance, accept ribs, while cervical (neck) vertebrae and lumbar vertebrae do not. The type of vertebra produced is specified past the Hox genes expressed in the somite.
1 of the most radical alterations of the vertebrate body plan is seen in the snakes. Snakes evolved from lizards, and they appear to accept lost their legs in a ii-footstep process. Both paleontological and embryological evidence supports the view that snakes kickoff lost their forelimbs and later lost their hindlimbs (Caldwell and Lee 1997; Graham and McGonnell 1999). Fossil snakes with hindlimbs, only no forelimbs, have been constitute. Moreover, while the most derived snakes (such as vipers) are completely limbless, more primitive snakes (such as boas and pythons) have pelvic girdles and rudimentary femurs.
The missing forelimbs can exist explained by the Hox expression blueprint in the anterior portion of the snake. In almost vertebrates, the forelimb forms but inductive to the most anterior expression domain of Hoxc-6 (Gaunt 1994; Shush et al. 1995). Caudal to that point, Hoxc-6, in combination with Hoxc-viii, helps specify vertebrae to be thoracic. During early python development, Hoxc-half-dozen is not expressed in the absenteeism of Hoxc-8, and then the forelimbs do not form. Rather, the combination of Hoxc-6 and Hoxc-8 is expressed for nearly of the length of the organism, telling the vertebrae to grade ribs throughout almost of the body (Figure 22.nine; Cohn and Tickle 1999).

Figure 22.9
Loss of limbs in snakes. (A) Skeleton of the garter ophidian, Thamnophis, stained with alcian blue. Ribbed vertebrae are seen from the head to the tail. (B, C) Hox expression patterns in chick (B) and python (C). (Photograph courtesy of A. C. Burke; B, C (more...)
The hindlimb buds do brainstorm to form in pythons, but they practice not make anything more a femur. This appears to be due to the lack of sonic hedgehog expression by the limb bud mesenchyme. Sonic hedgehog is needed both for the polarity of the limb and for maintenance of the apical ectodermal ridge (AER). Python hindlimb buds lack the AER. Interestingly, the phenotype of the python hindlimb resembles that of mouse embryos with loss-of-part mutations of sonic hedgehog (Chiang et al. 1996).
Hox genes and atavisms
Equally nosotros have seen, disruptions of the Hox genes can change one blazon of vertebra into another blazon. In some instances, the mutation of a Hox cistron tin can produce "atavistic" conditions, wherein the organism resembles an earlier evolutionary phase. Deletion or misregulation of the Hoxa-2 genes in mice, for example, results in a fractional transformation of the 2d pharyngeal arch into a re-create of the showtime pharyngeal arch. The mutant fetuses lack the stapes and styloid bones formed from the second arch, but accept extra malleus, incus, tympanic, and squamosal bones. They too possess a rodlike cartilage that has no counterpart in normal mice, only looks like the pterygoquadrate cartilage thought to accept been present in therapsids, the reptilian ancestors that gave ascension to the mammals (Figure 22.10; Rijli et al. 1993; Lohnes et al. 1994; Marking et al. 1995). Thus, major evolutionary changes tin be correlated with the alteration of Hox cistron expression in different parts of the embryo.
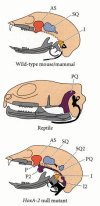
Figure 22.10
Representation of skeletal elements derived from the first pharyngeal curvation (in grey) and the second pharyngeal curvation (in black). (Every bit, alisphenoid; I, incus; I2, duplicated incus; P and P2, normal and duplicated pteroid cartilage; PQ, pterygoquadrate cartilage; (more than...)
Changes in Hox gene number
The number of Hox genes may play a function in permitting the evolution of circuitous structures. All invertebrates have a single Hox complex per haploid genome. In the most simple invertebrates—such as sponges—at that place announced to be just one or two Hox genes in this complex (Degnan et al. 1995; Schierwater and Kuhn 1998). In the more complex invertebrates, such as insects, at that place are numerous Hox genes in this complex. Comparing the Hox genes of chordates, arthropods, and molluscs suggests that at that place was a common set of vii Hox genes in the Urbilaterian ancestor of the protostomes and deuterostomes. Indeed, in invertebrate deuterostomes (echinoderms and amphioxus, an invertebrate chordate), at that place is only one Hox complex, which looks very much similar that of the insects (Effigy 22.11; Holland and Garcia-Fernández 1996). Past the fourth dimension the earliest vertebrates (agnathan fishes) evolved, at that place were at least four Hox complexes. The transition from amphioxus to early on fish is believed to be i of the major leaps in complexity during evolution (Amores et al. 1998; Holland 1998). This transition involved the evolution of the head, the neural crest, new cell types (such as osteoblasts and odontoblasts), the brain, and the spinal cord. As nosotros saw in Chapter 11, the regionalization of the brain and spinal cord is dependent upon Hox genes, and the regional specification of the somitic segments depends upon the paralogous members of the different Hox clusters. For instance, deletions of Hoxa-3 (from the A cluster) affect the neural crest-derived glands of the neck; deletions of Hoxd-3 (its paralogue from the D cluster) touch the somite-derived skeleton of the neck. This distinction may be due to the different levels of expression of these genes within the same tissues (Greer et al. 2000). Holland (1998) speculates that the generation of these new structures was allowed past the fourfold duplication of the Hox gene complex.

Figure 22.11
Postulated ancestry of the homeotic genes from a hypothetical ancestor of both deuterostomes and prototomes. Amphioxus has but one cluster, similar to that of insects. Vertebrates accept four clusters, none of which is complete. (Afterwards Holland and Garcia-Fernández (more than...)
![]()
Box
Assessing Homologies through Regulatory Factor Expression Patterns.
- *
-
The reason for this remarkable conservation of structure in the Hox gene complex is thought to be the sharing of cis-regulatory regions by neighboring genes. If a Hox cistron is moved to a unlike region within the complex, its regulation is altered. The critical regulatory regimes might be the binding sites for the Polycomb proteins. These proteins are also conserved throughout development, and they silence the Hox genes at specific times and places. Hither, and so, we meet a "phyletic constraint" at the molecular level (Chiang et al. 1995; Müller et al. 1995; Kmita et al. 2000).

Source: https://www.ncbi.nlm.nih.gov/books/NBK9978/
Posted by: worrellhavoing69.blogspot.com

0 Response to "What Animals Are Hox Genes Found In"
Post a Comment